Bonded Warehouse: Safe Storage for Healthcare Products

Bonded Warehouse: Compliance and Efficiency for Medical Devices Storing imported medical devices requires technical structure, strict management, and regulatory compliance. QR Group’s bonded warehouse service offers complete support from receipt to release for commercialization, ensuring that products are under strict control, either at the appropriate temperature or in safe areas, as required by ANVISA and […]
QR Group Explains Distribution Operations Management: What Medical Device Distributors Need to Know

Distribution Operations Management: control and efficiency in the logistics chain Managing the inventory and distribution of medical devices in Brazil requires complete visibility into inventory levels, accurate tracking, and efficient execution. QR Group offers a specialized service in the management of distribution operations, integrating technology, compliance and logistics control to ensure that your products arrive […]
Anvisa Prioritizes Registration of Tests for Arboviruses, Including Dengue and Chikungunya
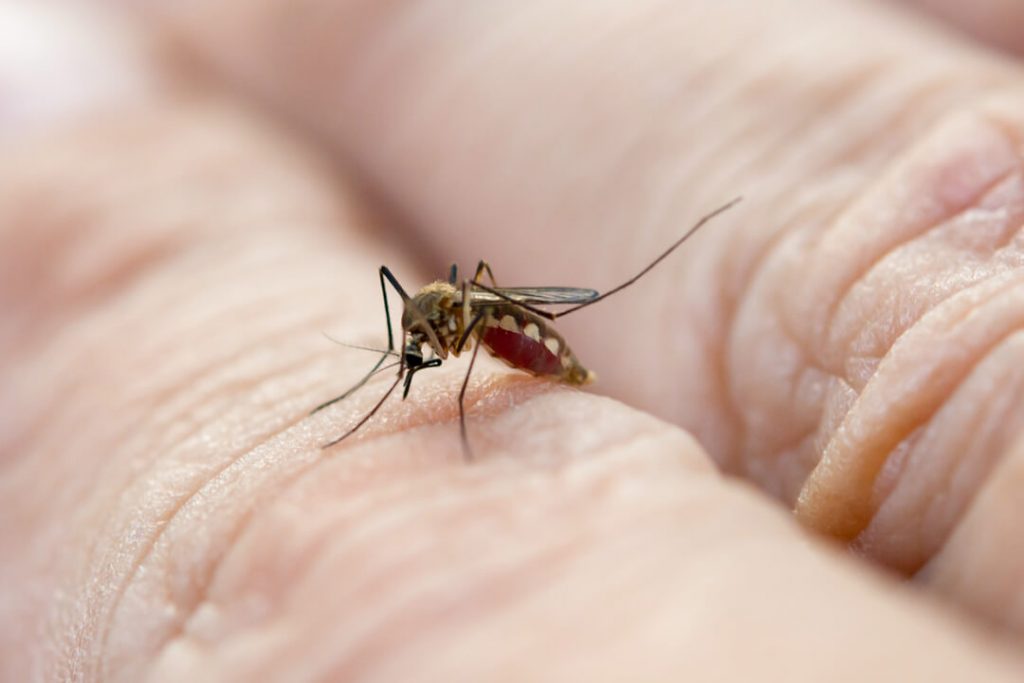
Anvisa is implementing measures to speed up the registration of diagnostic tests for mosquito-borne diseases, aiming at better control of arboviruses in Brazil. The National Health Surveillance Agency (Anvisa) recently announced that it will prioritize the analysis of requests for registration of tests for the diagnosis of mosquito-borne diseases, such as dengue, chikungunya and oropouche […]
Challenges for companies and inspectors in interpreting the requirements of legislation and international standards of Health Surveillance

One of the biggest challenges for companies that manufacture or import/distribute health products is understanding and/or interpreting regulatory requirements, whether they are resolutions/legislation or international standards of the Health Surveillance Agency. The applicability of certain requirements in certain segments must be carefully assessed by both companies and authorities, which makes the inspection process by health […]
Registering Medical Products in Brazil – What do you need to know?

Registering medical products in Brazil goes through ANVISA – the National Health Surveillance Agency – which is the agency responsible for approving the marketing of any product related to human health in Brazil. The agency divides products into categories, such as health products, cosmetics, cleaning products, medicines, food, cannabis products and tobacco. The first step […]
Anvisa Publishes English Version of IN 289/2024: Regulatory Convergence and Efficiency

How IN 289/2024 Facilitates the Registration and Post-Registration Process for Medicines and Biological Products in Brazil Anvisa has just released the English version of Normative Instruction (IN) 289/2024, reinforcing the agency’s commitment to international regulatory convergence. The new standard establishes procedures to streamline the registration and post-registration analysis of medicines, biological products, vaccines and Cadifas. […]
In Vitro Diagnostic Devices: Update on ANVISA’s Petitioning System under RDC 830/2023

QR Group provides guidance to international manufacturers on new regulatory requirements for in vitro diagnostic devices and how to adapt to RDC 830/2023 ANVISA will carry out an important update to its electronic petitioning system between May 29 and June 3 to comply with RDC 830/2023, which establishes new rules for the risk classification, registration, […]