Elevidys® Approval by ANVISA: The Impact of Gene Therapy for Duchenne Muscular Dystrophy
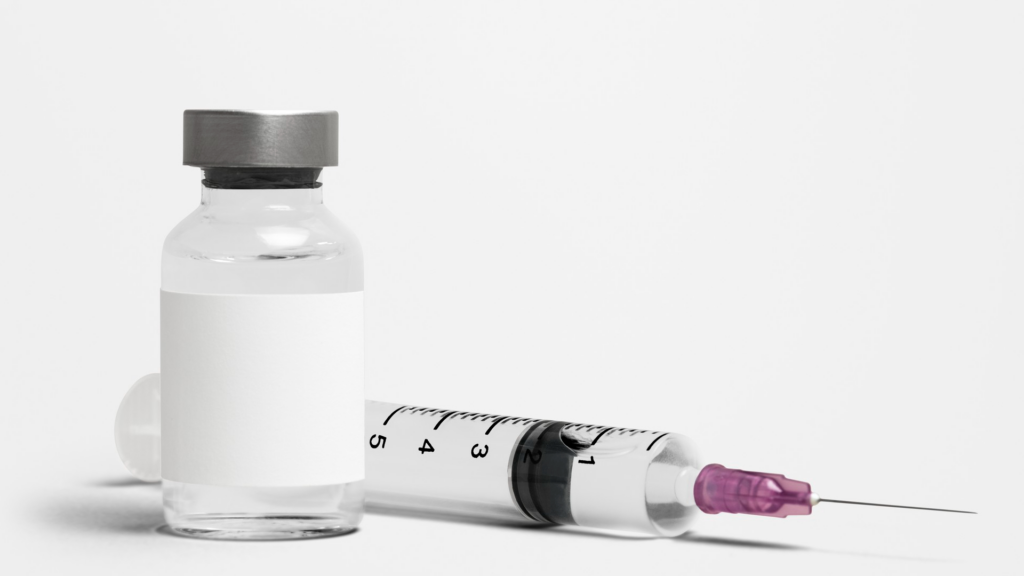
Understand the Approval of Elevidys and How QR Group Assists® in the Registration Process of Innovative Products in Brazil The approval of Elevidys® (delandistrogen moxeparvoveque) by ANVISA for the treatment of Duchenne Muscular Dystrophy (DMD) represents a milestone in the area of advanced therapies in Brazil. QR Group, with its expertise in medical device and […]
QR Group Explains Anvisa’s New Rules for Importing Medical Device Components

Understand how the updates to RDCs 860/2024 and 939/2024 impact the import and storage of medical device components and accessories in Brazil Anvisa has updated the processes and flows related to the import and storage of medical device components. With the recent publication of Collegiate Board Resolutions (RDCs) 860/2024 and 939/2024, importers need to understand […]
Update on Smoking Product Registration and Renewal: What Companies Need to Know

Understand how the update of ANVISA’s guidelines affects the registration and renewal of smoking products and how QR Group can help in the process. ANVISA recently updated its guidelines on the registration and renewal of smoking products derived from tobacco. This change is included in the Collegiate Board Resolution (RDC) 896/2024, which reformulates the requirements […]
ANVISA’s Participation in WHO: Impacts on the Regulation of Medical Products

Understand the Importance of ANVISA’s Role in the Global Product Regulation ANVISA is participating in the 13th plenary meeting of the Member States Mechanism on Substandard and Falsified Medical Products (MSMSF) of the World Health Organization (WHO), reinforcing its essential role in product regulation at the international level. The event will take place between November […]